Quality Control Standards in Aerosol Manufacturing: 2024 Complete Guide
According to recent industry data, over 40% of aerosol manufacturing quality issues stem from inadequate testing protocols. At Signature Filling Company, our rigorous quality control standards have helped maintain a 99.9% product acceptance rate, significantly above the industry average of 95%.
Aerosol Manufacturing Quality Control Standards
Quality control in aerosol manufacturing has evolved significantly, with the global aerosol testing market reaching $2.3 billion in 2023. Modern standards incorporate:
Physical Testing Requirements
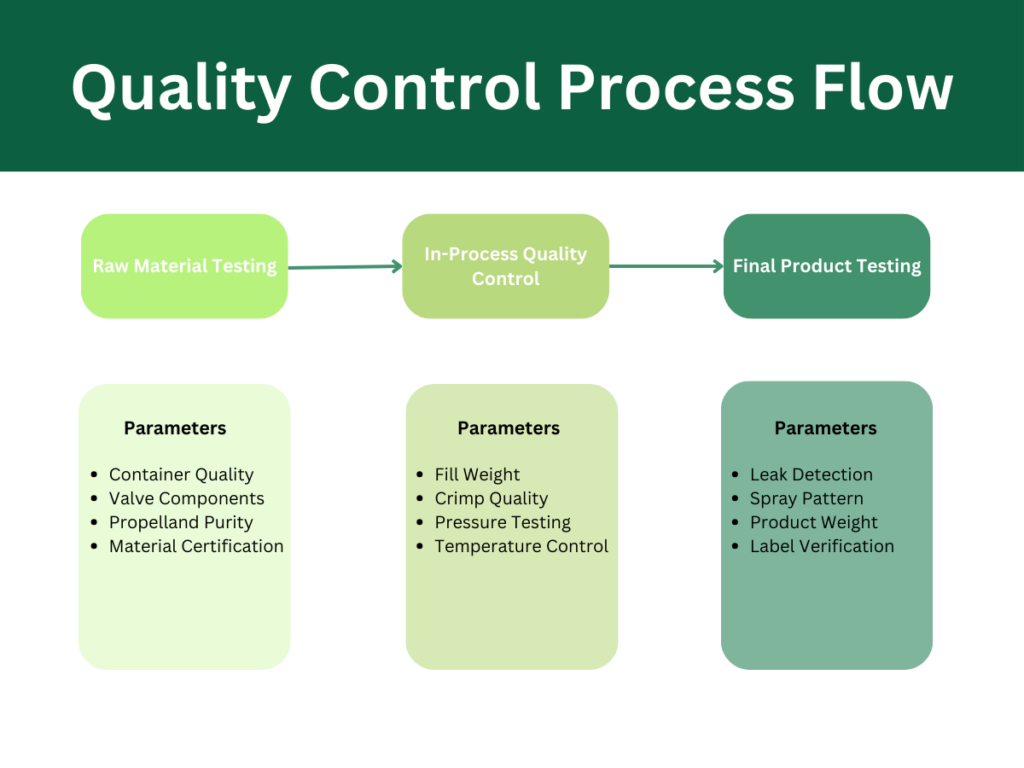
- Leak Detection Testing (99.99% accuracy required)
- Pressure Verification (±2% tolerance)
- Spray Pattern Analysis (180° coverage testing)
- Container Integrity Verification (5,000+ PSI testing)
Chemical Analysis Protocols
Industry statistics show that chemical analysis accounts for 35% of quality control procedures:
- Propellant Composition Testing
- Active Ingredient Concentration Analysis
- pH Level Monitoring (±0.1 accuracy)
- Stability Testing (accelerated and real-time)
Essential Aerosol Testing Equipment and Procedures
Modern quality control relies on precision equipment, representing an average investment of $500,000 for a fully-equipped testing facility:
Advanced Testing Infrastructure
- Electronic Leak Detection Systems (99.99% detection rate)
- Automated Vision Inspection Equipment
- High-Precision Weight Control Systems (±0.01g accuracy)
- Environmental Testing Chambers
Compliance Requirements in Aerosol Quality Control
Quality control compliance combines multiple regulatory frameworks:
Industry Standards Comparison Table
| Standard Type | Requirements | Testing Frequency | Documentation |
|---|---|---|---|
| FDA cGMP | Full batch testing | Every production run | Complete batch records |
| EPA Standards | Environmental impact | Monthly | Emissions reports |
| ISO 9001 | Process validation | Quarterly | Audit reports |
Quality Control Implementation Guide
Based on Signature Filling Company’s experience with over 10,000 successful production runs:
- Raw Material Inspection
- Container quality verification (0.01% defect tolerance)
- Valve component testing (100% inspection rate)
- Propellant purity checks (99.99% minimum)
- In-Process Controls
- Continuous monitoring systems
- Real-time data analytics
- Automated quality alerts
Industry Best Practices and Future Trends
Recent industry research indicates:
- 60% of manufacturers are implementing AI-driven quality control
- 45% reduction in defects through automated testing
- 30% efficiency improvement with integrated quality systems
Sustainability Integration
At Signature Filling Company, our sustainable quality control measures include:
- Zero-waste testing procedures
- Energy-efficient equipment
- Recycled material verification
- Environmental impact monitoring
Frequently Asked Questions
Q: What are the main quality control tests for aerosol products? A: Essential tests include leak detection, pressure testing, spray pattern analysis, and chemical composition verification. Industry standards require a minimum of 12 different quality control checkpoints during production.
Q: How often should aerosol manufacturing equipment be tested? A: Equipment calibration and testing should occur daily for critical parameters, with comprehensive testing weekly. Signature Filling Company implements continuous monitoring with hourly verification checks.
Q: What are the FDA requirements for aerosol quality control? A: FDA requirements include complete batch documentation, validated testing procedures, and regular equipment calibration. All processes must follow current Good Manufacturing Practices (cGMP).
Conclusion and Next Steps
Quality control in aerosol manufacturing requires precision, expertise, and state-of-the-art equipment. With over two decades of experience and a proven track record of excellence, Signature Filling Company maintains superior quality standards that exceed industry requirements.
Take Action Today
Ready to experience industry-leading quality control in your aerosol manufacturing?
✓ 99.9% Product Acceptance Rate ✓ ISO 9001 Certified Facility ✓ FDA/EPA Compliant Processes ✓ 24/7 Quality Monitoring
